Imagine a fascinating world of bustling research labs and friendly corner pharmacies, where a crucial discipline works its magic to bring healing and better health on our never-ending journey of wellness. Behind the scenes of every prescription, there lies an unsung hero safeguarding public health and protecting patients from potential risks – Pharmacovigilance.
Pharmacovigilance is like a trustworthy guardian in the pharmaceutical world, always keeping a close eye on drug safety. By constantly watching out for and stopping any harmful drug reactions, it puts patients’ well-being first in the ever-evolving field of medicine. It operates with a clear mission: to detect and prevent adverse drug reactions, making patient safety its unwavering priority. By meticulously gathering and analyzing data on medication use, pharmacovigilance experts strive to identify potential risks and swiftly take action, fostering a world where pharmaceuticals heal without harm.
Just join me on a captivating journey as we explore the wonders of pharmacovigilance – the guardian of our well-being. Together, we’ll uncover the secrets behind its crucial role in detecting and preventing adverse drug reactions. Get ready to dive into the fascinating world of data analysis, reporting, and collaboration—a powerful shield against the unexpected!
Table of Contents
What is Pharmacovigilance?
Pharmacovigilance is the vigilant practice of monitoring and ensuring the safety of pharmaceutical products after approval. Its purpose is to detect, assess, and prevent adverse drug reactions (ADRs) during real-world usage.
Pharmacovigilance serves as a sentinel, diligently scrutinizing medications even after they have received regulatory approval. While clinical trials provide essential safety data during drug development, they may not always capture the full spectrum of adverse reactions that can occur in real-world scenarios. Once a drug is out there, pharmacovigilance takes charge, monitoring its safety in different patients and situations. It carefully examines reported side effects, assessing risks non-stop. When potential safety concerns arise, quick and efficient investigations are launched, and the findings are promptly communicated to healthcare professionals and the public.
Pharmacovigilance doesn’t stop after one try; it’s an ongoing dedication to your safety. Monitoring medication safety is a continuous journey that lasts from the drug’s approval to its real-world use. Your well-being remains a top priority throughout the entire lifecycle of the product. In summary, pharmacovigilance is a crucial defender, fully devoted to patient safety and the pharmaceutical industry’s integrity. With vigilant monitoring and ongoing assessments, it’s shaping a future where medical advancement is guided by compassion and a strong commitment to public well-being.

The Lifesaving Importance of Pharmacovigilance: Why it Truly Matters!
Medications are designed to heal, but like any adventure, they come with risks. Despite rigorous pre-approval clinical trials, some unexpected reactions may emerge only when a drug is in the hands of countless patients. This is where the power of post-marketing surveillance through pharmacovigilance becomes indispensable—it’s our shield against unforeseen dangers, ensuring your well-being is protected every step of the way.
Pharmacovigilance plays a pivotal role in building comprehensive drug safety profiles. By continuously monitoring real-world data, it uncovers patterns and associations between medications and adverse events. This ongoing evaluation allows for a better understanding of a medication’s safety profile in diverse patient populations.
Throughout the ages, we’ve encountered pivotal moments that underscore the critical role of pharmacovigilance. Take the haunting thalidomide tragedy of the 1950s and 1960s, which tragically caused severe birth defects. Thanks to pharmacovigilance’s rapid response in connecting thalidomide to these devastating effects, it sparked vital regulatory changes and reinforced the importance of unwavering post-marketing surveillance. Together, we’re building a safer future for all.
Another example is the discovery of adverse cardiac effects associated with certain anti-inflammatory medications, which led to label warnings and heightened monitoring. In each instance, pharmacovigilance has responded decisively to safeguard patient well-being and ensure that the benefits of medications far outweigh potential risks.
Detecting the Unseen: Unraveling the Process of Pharmacovigilance
Pharmacovigilance follows a systematic approach to gather safety data from various sources, including healthcare professionals, patients, and regulatory agencies. This method involves using structured systems to report adverse events and potential drug-related problems, ensuring standardized data collection.
Think of pharmacovigilance as a safety net, constantly gathering valuable insights from doctors, nurses, patients, and regulatory authorities. It’s a coordinated effort to analyze patterns and trends, catching any potential safety concerns early on. This vigilance allows timely interventions and regulatory actions, maintaining trust in the pharmaceutical industry and assuring patients that their well-being remains the top priority.
Steps involved in Pharmacovigilance
Adverse Event Reporting
Encouraging healthcare professionals, patients, and consumers to report medication-related issues, providing vital details for enhanced pharmacovigilance.
Data Collection and Entry
Experts gather and review safety data from diverse sources – spontaneous reports, clinical trials, scientific literature, and patient support programs – entered into databases for in-depth analysis.
Data Analysis and Signal Detection
Pharmacovigilance experts analyze data using sophisticated techniques to identify patterns, trends, and potential signals for further investigation.
Signal Validation
Identified signals undergo strict validation to confirm accuracy, clinical relevance, and strength of association, considering biological plausibility and event consistency.
Assessing Risks and Casuality
Validated signals prompt in-depth risk assessment to evaluate medication-adverse event causality, utilizing tools like WHO-UMC causality categories for likelihood determination.
Benefit-Risk Assessment
After causality evaluation, a benefit-risk analysis is conducted to weigh medication benefits against potential risks, guiding regulatory decisions and safety measures.
Tripartite Collaboration in Pharmacovigilance
Pharmacovigilance isn’t a one-person show; it’s a dynamic collaboration among regulatory agencies, healthcare professionals, and pharmaceutical companies. With each player performing a vital role, this united front ensures medication safety is at its finest.
Regulatory agencies set the stage, establishing guidelines and overseeing the vigilant activities of pharmacovigilance. Meanwhile, healthcare professionals step into the spotlight, reporting adverse events and providing invaluable clinical insights. Pharmaceutical companies take the stage too, monitoring safety data and adhering to strict regulatory requirements. Together, they form a formidable network—a watchful eye that rapidly identifies and tackles potential safety concerns.
United through shared responsibilities and open communication, these stakeholders work tirelessly to uphold the highest standards of drug safety and protect public health. With this collaborative spirit, you can rest assured that your well-being remains the top priority in the world of medications. The power of teamwork shines brightly, keeping you safe and sound on your journey to better health.”
Reporting and Communication in Pharmacovigilance
Both healthcare professionals and patients share a vital duty of reporting adverse events promptly and accurately. This reporting is essential for pharmacovigilance, ensuring better drug safety. Professionals’ expertise adds crucial medical context, while patients’ firsthand experiences offer unique perspectives, shaping a thorough safety assessment.
Pharmacovigilance info reaches far and wide through diverse channels. The medical community stays informed via scientific publications, conferences, and specialized platforms. Healthcare pros receive safety updates, helping them make informed treatment decisions for better patient care. For the public, pharmacovigilance information is accessible through public health websites, patient leaflets, and direct-to-consumer communications. The aim is to empower patients with crucial safety details, encourage reporting of adverse events, and deepen their understanding of medication safety.
Safeguarding Lives: Pharmacovigilance Issues Vital Safety Alerts and Warnings
Pharmacovigilance info reaches far and wide through diverse channels. The medical community stays informed via scientific publications, conferences, and specialized platforms. Healthcare pros receive safety updates, helping them make informed treatment decisions for better patient care.
In some cases, safety alerts are issued, informing healthcare professionals and the public about potential risks associated with specific medications. These alerts may include updated prescribing information, contraindications, or precautions to ensure safer use. For the public, pharmacovigilance information is accessible through public health websites (eg. PvPI), patient leaflets, and direct-to-consumer communications. The aim is to empower patients with crucial safety details, encourage reporting of adverse events, and deepen their understanding of medication safety.
Ensuring Drug Safety in Future
Pharmacovigilance's Evolving Landscape: Embracing Data Analysis & Tech Advancements
The landscape of pharmacovigilance is continuously evolving, driven by advancements in data analysis and technology. With the increasing volume of safety data, artificial intelligence and machine learning algorithms are being leveraged to expedite signal detection and identify previously unnoticed patterns. Data mining techniques enable pharmacovigilance experts to sift through vast datasets efficiently, leading to quicker identification of potential safety signals and improved patient safety.
Global Efforts to Enhance Drug Safety Through International Collaborations
Recognizing the importance of global cooperation, international collaborations have become integral to enhancing drug safety. Regulatory agencies, pharmaceutical companies, and healthcare professionals across countries work together to share safety data, harmonize reporting standards, and strengthen pharmacovigilance infrastructure. Collaborative initiatives allow for the pooling of resources and expertise, enabling faster detection and response to global safety issues, ultimately benefitting patients worldwide.
Potential Impact of Pharmacovigilance on Future Drug Development and Patient Care
Pharmacovigilance’s impact on future drug development and patient care is immense. As a growing repository of real-world safety data accumulates, pharmacovigilance contributes valuable insights to drug development processes. It aids in refining risk-benefit profiles, guiding researchers toward safer medication formulations, and identifying specific patient populations that may benefit most from certain drugs.
Moreover, pharmacovigilance’s proactive approach to monitoring medication safety has the potential to transform patient care. Early detection and management of adverse reactions can lead to timely interventions, reducing the burden of medication-related harm and improving treatment outcomes. Pharmacovigilance’s role in shaping evidence-based prescribing practices and promoting a culture of patient safety are invaluable contributions to the future of healthcare.
In conclusion, the future of pharmacovigilance holds great promise. With technology-driven advancements and collaborative efforts, this vital discipline will continue to enhance drug safety, inform drug development, and protect patients worldwide. As we navigate the ever-changing landscape of healthcare, pharmacovigilance remains steadfast in its commitment to ensuring that every step we take towards better health is one that prioritizes patient well-being and safety.
Concluding the Chapter: The Power of Pharmacovigilance
In conclusion, pharmacovigilance stands as a formidable shield, safeguarding drug safety and upholding public health with unwavering dedication. This critical discipline plays a pivotal role in the post-marketing surveillance of pharmaceutical products, ensuring that the benefits of medications continue to outweigh potential risks.
By systematically collecting and analyzing safety data, pharmacovigilance detects and assesses adverse drug reactions, uncovering vital insights that shape our understanding of medication safety. Through international collaborations and advancements in data analysis and technology, pharmacovigilance has transcended geographical boundaries, fostering a global network that reinforces patient protection.
As we journey into the future of healthcare, the importance of active engagement in drug safety cannot be understated. I encourage readers to be proactive advocates for their well-being by promptly reporting any observed or suspected adverse events to healthcare professionals or relevant authorities. Staying informed about drug safety information empowers us to make informed decisions about our health and contributes to a collective commitment to safer medication use.
This introductory exploration of pharmacovigilance merely scratches the surface of its multifaceted nature. In upcoming blog posts, we will delve deeper into specific aspects of pharmacovigilance, shedding light on its evolving landscape, the intricacies of data analysis, and the impact of proactive safety measures on patient care.
Let us embrace the future of pharmacovigilance with unwavering vigilance, knowing that each step we take in ensuring drug safety is a step towards a healthier, safer tomorrow for all. Together, let us celebrate the unsung heroes of pharmacovigilance, whose tireless efforts are instrumental in preserving the trust and well-being of patients worldwide.
We would love to hear from you! Share your valuable thoughts and experiences related to drug safety and pharmacovigilance in the comments section below. Your insights play a vital role in fostering a community committed to patient well-being and advancing healthcare.
Stay informed and stay ahead! Subscribe to our blog for regular updates on future articles delving deeper into various aspects of the pharmaceutical industry. By subscribing, you’ll gain access to exclusive content that empowers you to make informed decisions about your health and stay abreast of the latest developments in drug safety.
Together, let’s build a safer and healthier tomorrow through active engagement in the journey of pharmacovigilance and a shared commitment to protecting public health. Join us on this transformative path towards a future of medication excellence and patient-centric care. For pharmacovigilance books required for pharmacy courses, one can visit here.
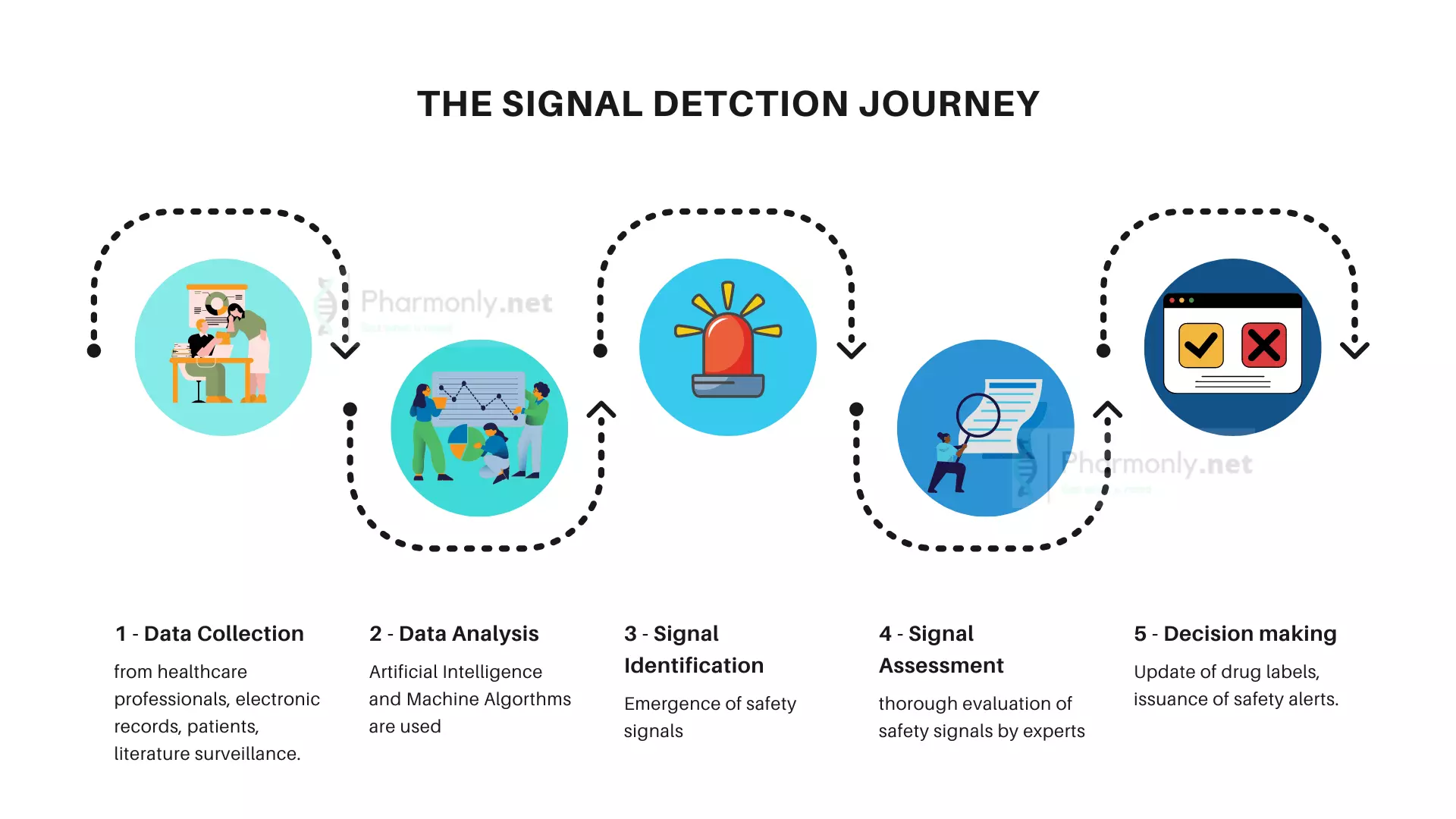
Pharmacovigilance Methods and Signal Detection
Continue reading series.

Pingback: Pharmacovigilance Methods and Signal Detection
Pingback: Guidelines for Pharmacovigilance and Global Collaboration
Pingback: Future of Pharmacovigilance - Advancements in Drug Safety