Pharmacovigilance is a critical discipline in the pharmaceutical industry dedicated to monitoring and ensuring the safety of medications after they are approved for use. It employs a range of methodologies to detect and analyze adverse drug reactions (ADRs) to protect public health. Curious to Dive Deeper into Pharmacovigilance basics? Check Out Our Intro to Pharmacovigilance!

More about basics of Pharmacovigilance
Before proceeding, one should know basics of pharmacovigilance.
Table of Contents
Introduction to Pharmacovigilance Methods
Pharmacovigilance employs a range of dynamic methods to ensure medication safety. Spontaneous reporting involves healthcare professionals and patients reporting adverse events, providing crucial real-world data. Literature surveillance scours scientific publications for valuable insights. Electronic health records analysis digs into vast medical databases to detect potential signals. Social media monitoring keeps a vigilant eye on public sentiments and emerging safety concerns. Together, these methods create a robust system, ensuring comprehensive surveillance and safeguarding public health.
Throughout this captivating blog post, we shall embark on a journey of discovery, delving into the myriad of methods harnessed by pharmacovigilance. Each method is a unique and essential contributor to the all-encompassing watchfulness over drug safety, ensuring that every aspect of your well-being is protected with utmost care. Let’s unravel the secrets of how this critical discipline works tirelessly to safeguard your health, revealing the hidden world of pharmacovigilance like never before.
Spontaneous Reporting System: Empowering Healthcare
In the realm of pharmacovigilance, the spontaneous reporting system stands as a crucial mechanism for identifying and evaluating adverse drug reactions (ADRs). This system relies on the voluntary reporting of suspected ADRs by healthcare professionals and patients, empowering them to contribute actively to drug safety surveillance.
The spontaneous reporting system is a cornerstone of pharmacovigilance, designed to capture unexpected and rare adverse events that may not have been detected during clinical trials. It allows healthcare professionals and patients to report their observations of suspected ADRs voluntarily. This reporting process is initiated when a healthcare professional or patient identifies an adverse event that might be linked to a specific medication. The key components of the spontaneous reporting system include:
Voluntary Reporting
The essence of the spontaneous reporting system lies in its voluntary nature. Healthcare professionals, such as physicians, nurses, and pharmacists, as well as patients themselves, can choose to report suspected adverse reactions without any legal obligations. This voluntary approach encourages reporting, as it removes any fear of consequences or repercussions for reporting ADRs.
Reporting Forms and Tools
Pharmacovigilance centers provide standardized reporting forms and tools to facilitate the collection of information about suspected ADRs. These forms often include details about the patient, the suspected drug, the observed adverse event, and relevant medical history. Seamlessly contributing to the process, healthcare professionals and patients alike play a vital role in keeping medications safe and your well-being a top priority.
Centralized Reporting Centers
Each country typically has a national pharmacovigilance center that acts as a centralized repository for all spontaneous reports. Healthcare professionals and patients can submit their reports directly to these centers through various channels, such as online portals, email, or postal services. The national centers then analyze and assess the reported data collectively.
The information collected through spontaneous reports aids in the continuous improvement of medication safety, ultimately safeguarding public health.
Literature Survey and Database Analysis: Uncovering Safety Signals
In the dynamic field of pharmacovigilance, harnessing the power of scientific literature and vast databases is instrumental in identifying potential safety signals associated with medications. This section focuses on how pharmacovigilance experts systematically review medical literature and databases to ensure a comprehensive understanding of drug safety profiles.
Literature Surveillance in Pharmacovigilance
Literature surveillance involves the systematic examination of published scientific articles, clinical trials, case reports, and medical conference proceedings to identify information related to adverse drug reactions (ADRs). Pharmacovigilance experts conduct exhaustive literature searches using databases such as PubMed, Embase, and other academic resources. The process includes three following steps.
Identifying relevant studies
Pharmacovigilance experts meticulously search for articles and studies that mention specific medications or drug classes. They look for data related to known and potential safety concerns, especially those not previously captured during clinical trials.
Analyzing case reports
Case reports provide invaluable insights into rare and unusual adverse events. Pharmacovigilance experts analyze individual case reports to identify patterns or clusters of ADRs that might suggest a potential safety signal.
Reviewing clinical trials data
Pharmacovigilance professionals scrutinize clinical trial data, especially those from post-marketing studies, to assess the drug’s safety in real-world patient populations. They compare the reported adverse events with the ones observed during the pre-marketing clinical trials.
Database Analysis in Pharmacovigilance
Pharmacovigilance experts tap into a wealth of knowledge through comprehensive databases that encompass electronic health records, claims databases, and other real-world data sources. These invaluable resources empower them to stay ahead in the quest for drug safety, ensuring your well-being remains at the forefront of medical advancements. The process includes three steps viz.
Real World Evidence Analysis
Real-world data from electronic health records and claims databases offer a wealth of patient information beyond what is captured in controlled clinical trials. Pharmacovigilance experts analyze this data to detect potential safety signals and assess the drug’s performance in diverse patient populations.
Data Mining Techniques
With advanced data mining techniques at their fingertips, pharmacovigilance experts delve into vast databases, unearthing valuable patterns, trends, and connections that could signify potential safety concerns. These cutting-edge methods prove invaluable in spotting adverse drug reactions (ADRs) that might have eluded traditional detection.
Pharmacovigilance Signal Detection Algorithms
The experts develop and apply signal detection algorithms to database analysis. These algorithms automatically process data and highlight potential safety signals for further investigation.
Literature surveillance and database analysis take the stage, forming a rock-solid foundation for unearthing potential safety signals tied to medications. With systematic reviews of medical literature and real-world databases, we gain deeper insights into drug safety profiles, leading to better patient outcomes and a healthier society.
Signal Detection and Data Mining: Unveiling Safety Patterns
Signal detection and data mining stand at the forefront of pharmacovigilance efforts, employing sophisticated statistical and analytical methods to unveil potential safety signals associated with medications. In this section, we will delve into the significance of these techniques in identifying adverse drug reactions (ADRs) and their impact on ensuring drug safety.
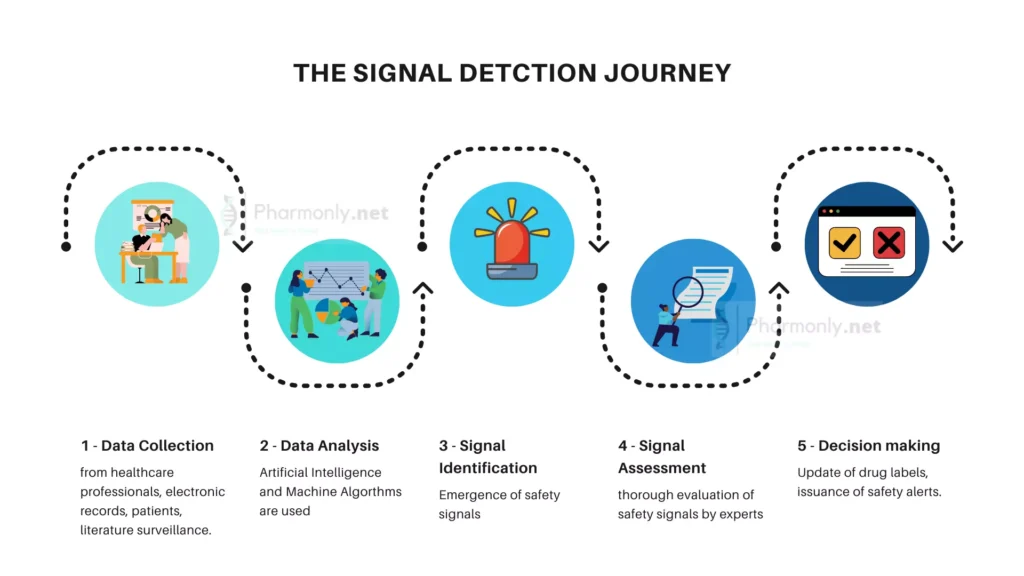
Signal detection in Pharmacovigilance
Signal detection involves the systematic analysis of pharmacovigilance data to identify patterns or associations that may indicate potential safety issues with specific medications. Pharmacovigilance experts employ the following approaches in signal detection:
Quantitative Methods
Pharmacovigilance professionals use various quantitative methods, such as the proportional reporting ratio (PRR), the reporting odds ratio (ROR), and the Bayesian confidence propagation neural network (BCPNN), to calculate statistical measures that reveal potential signals. These methods compare the number of observed adverse events for a particular drug with the number expected based on the drug’s usage, helping identify disproportionate reporting patterns.
Temporal Analysis
Pharmacovigilance experts analyze the time elapsed between drug exposure and the occurrence of adverse events. Clustering of adverse events shortly after drug initiation or discontinuation might indicate potential safety concerns.
Data mining techniques in Pharmacovigilance
Data mining techniques play a pivotal role in pharmacovigilance, enabling the discovery of hidden patterns and relationships within large datasets. Common data mining techniques include:
Text Mining
Text mining involves extracting valuable information from unstructured data sources, such as medical narratives, case reports, and social media posts. By analyzing unstructured data, pharmacovigilance experts can identify ADRs that might not be present in structured databases.
Association rule mining
Association rule mining identifies relationships between different medical conditions, drug treatments, and adverse events. This technique helps pharmacovigilance professionals recognize potential co-occurrences of events that might warrant further investigation.
Clustering and pattern reconition
Clustering techniques group similar drugs or similar adverse events together, revealing patterns that may highlight potential safety signals.
Time Series Analysis
Time series analysis helps detect trends and patterns over time, enabling experts to identify seasonal variations or long-term changes in ADR reporting.
Signal detection and data mining techniques form the backbone of modern pharmacovigilance. By employing statistical analyses and advanced data mining methods, pharmacovigilance experts can effectively identify patterns and associations that may indicate potential safety issues with medications. These techniques aid in the early detection of adverse reactions, contributing to safer medications and improved patient care.
Signal Validation and Assessment: Ensuring Accurate Safety Findings
To ensure the accuracy and reliability of potential safety signals identified through various methods, a rigorous process of signal validation and assessment is employed. Signal validation is the process of examining potential safety signals identified through signal detection methods to determine if they are genuinely associated with a medication or merely chance occurrences. The assessment involves a series of systematic and scientific evaluations to ascertain the strength and validity of the signals.
Signal validation criteria
Pharmacovigilance experts establish specific criteria to validate potential safety signals. These criteria encompass factors such as the strength of the statistical association, the consistency of the signal across different data sources, the clinical plausibility of the observed adverse event, and the biological plausibility of the association.
Case confirmation
Each individual case included in the signal is carefully reviewed to confirm the reported adverse event and its association with the suspected medication. Pharmacovigilance professionals scrutinize medical records, laboratory data, and other relevant information to verify the accuracy of the reported ADR.
Dose-response relationship
The existence of a dose-response relationship strengthens the validity of a potential safety signal. Pharmacovigilance experts assess if there is an increased risk of the adverse event with higher doses of the medication, supporting a causal relationship.
Casualty assessment
Pharmacovigilance experts employ various causality assessment tools, such as the Naranjo algorithm and the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) system, to evaluate the likelihood of the medication causing the adverse event.
Comparative analysis
Pharmacovigilance experts compare the observed rates of the adverse event with the background rates in the general population or the rates observed with other similar medications. This comparative analysis aids in distinguishing true safety signals from background noise.
Expert review and consensus
The validation process often involves multiple experts reviewing the data independently. Through collaboration and consensus-building, the strength and validity of the signal are determined.
Challenges and Limitations of Signal Detection in Pharmacovigilance
In the world of pharmacovigilance, signal detection is like hunting for hidden treasure, but it comes with its fair share of challenges and limitations. Our vigilant experts brave obstacles to identify and assess potential safety signals tied to medications. In this section, we will address the key challenges and limitations faced in signal detection, including underreporting, confounding factors, and signal ambiguity.
- Underreporting of adverse events: One of the most significant challenges in pharmacovigilance is the underreporting of adverse events. Healthcare professionals and patients may be unaware of the reporting system or may not recognize the significance of reporting a suspected adverse reaction. Additionally, some adverse events may go unreported due to fear of legal consequences or lack of time to complete reporting forms.
- Voluntary nature of reporting: The voluntary nature of adverse event reporting can result in selective reporting bias. Some adverse events, especially common or well-known ones, may be underreported, while rare or severe adverse events may receive more attention. This can lead to an incomplete picture of the drug’s safety profile.
Confounding factors and Data noise: Signal detection in pharmacovigilance often involves analyzing real-world data, which can be noisy and affected by confounding factors. Other medications, underlying medical conditions, and lifestyle factors may influence the occurrence of adverse events, making it challenging to attribute the event solely to the suspected medication.
Signal Ambiguity: Not all signals identified during signal detection may represent true safety concerns. Some signals may be chance findings or related to factors other than the medication. Pharmacovigilance experts need to carefully assess and differentiate true safety signals from random fluctuations.
Low Reporting Rates for Rare Adverse Events: Rare adverse events are more challenging to detect due to their low reporting rates. Even if a potential safety signal is valid, it may take a more extended period or multiple reports before the signal gains statistical significance.
Data Quality and Completeness: The accuracy and completeness of the data collected during adverse event reporting impact the reliability of signal detection. Incomplete or inaccurate data can lead to misinterpretation and potentially compromise patient safety.
- Lack of Standardized Data Collection: The lack of standardized data collection across different healthcare settings and countries can hinder effective signal detection and analysis. Variability in data formats and definitions can make it challenging to compare safety data across regions.
Enhancing Signal Detection: The Role of Advanced Technology
In the ever-evolving landscape of pharmacovigilance, advanced technologies and innovative algorithms have emerged as powerful tools in enhancing signal detection. These cutting-edge approaches, such as artificial intelligence (AI) and machine learning (ML), are revolutionizing the way potential safety signals are identified and analyzed. AI and ML have rapidly gained prominence in various fields, including healthcare. In pharmacovigilance, these technologies have shown immense promise in revolutionizing signal detection by offering unprecedented capabilities to analyze vast amounts of data with speed and precision.
Automated Data Processing: AI and ML algorithms enable automated data processing, efficiently sifting through enormous volumes of structured and unstructured data. This streamlined approach significantly reduces the time required for signal detection and allows for real-time surveillance of drug safety.
Natural Language Processing (NLP): NLP is a branch of AI that empowers computers to understand human language. In pharmacovigilance, NLP algorithms extract valuable information from unstructured data sources, such as medical narratives, case reports, and scientific literature. This allows for a more comprehensive analysis of adverse events reported in various formats.
Early Signal Detection: AI and ML algorithms can detect signals at an earlier stage, even when the event occurrence is relatively rare. By identifying potential safety signals sooner, these technologies enable timely action to mitigate risks and enhance patient safety.
Real-Time Surveillance: The real-time capabilities of AI and ML ensure continuous monitoring of drug safety data, providing a proactive approach to signal detection. This real-time surveillance allows pharmacovigilance experts to promptly respond to emerging safety concerns.
Identifying Patterns and Associations: AI and ML excel at recognizing complex patterns and associations within large datasets. By identifying subtle relationships between medications and adverse events, these technologies can reveal safety signals that might have been missed by traditional statistical methods.
Adaptive Learning: Machine learning algorithms possess the ability to adapt and improve their performance based on new data and experiences. As more data becomes available, these algorithms refine their signal detection capabilities, leading to more accurate and reliable results over time.
Supporting Signal Validation: AI and ML can assist in the signal validation process by providing additional insights and data-driven evidence. The combination of advanced algorithms and expert ass
The Role of Pharmacovigilance Centers and Networks: Strengthening Global Networks
In the realm of pharmacovigilance, collaboration and information sharing are essential components in ensuring drug safety on a global scale. National and international pharmacovigilance centers play a crucial role in fostering cooperation among various stakeholders and facilitating the exchange of safety data. Pharmacovigilance centers serve as central hubs for collecting and analyzing adverse event reports associated with medications. They play a pivotal role in monitoring the safety profiles of drugs in their respective regions and act as vital conduits for communication and collaboration on an international level.
National Pharmacovigilance Centers
At the national level, each country typically has its own pharmacovigilance center responsible for managing and overseeing drug safety surveillance within its borders. National centers receive spontaneous adverse event reports from healthcare professionals and patients and compile safety data related to medications available in their country.
Collaborative Networks
National pharmacovigilance centers are often part of collaborative networks that facilitate the exchange of information and expertise between countries. Such networks may be regional, continental, or global in scope, allowing for seamless communication and cooperation on drug safety matters.
Data Sharing & Harmonization
Collaborative networks enable pharmacovigilance centers to share safety data and pool resources effectively. Data sharing allows for a broader and more diverse dataset, leading to improved signal detection and the identification of safety signals that may be relevant to multiple countries.
Early signal detection & Rapid communication
Through collaborative efforts, pharmacovigilance centers can collectively detect and assess potential safety signals more efficiently. This timely exchange of safety information enables rapid communication and action when addressing emerging safety concerns.
Knowledge and expertise sharing
Collaborative networks provide a platform for sharing knowledge and expertise in pharmacovigilance. This facilitates mutual learning, best practice sharing, and capacity building, ultimately enhancing the overall competency of drug safety surveillance worldwide.
Contribution to global pharmacovigilance database
International collaborations allow pharmacovigilance centers to contribute data to global pharmacovigilance databases. These databases serve as valuable resources for researchers, regulators, and healthcare professionals, providing a comprehensive view of drug safety at a global level.
Impact of Signal Detection on Patient Safety: A Pillar of Improved Outcomes
Signal detection serves as a crucial pillar in the realm of pharmacovigilance, ensuring early detection and assessment of potential safety concerns associated with medications. This proactive approach plays a pivotal role in enhancing patient safety and improving drug safety profiles. Early detection and assessment of potential safety signals are fundamental in pharmacovigilance, as they enable timely action and intervention. This proactive approach yields several key benefits that directly impact patient safety and drug safety profiles.
- Improved Patient Outcomes: Timely detection of potential safety signals allows healthcare professionals to be proactive in addressing adverse events promptly. Taking swift action to manage risks associated with medications can lead to better patient outcomes and prevent the escalation of adverse reactions.
- Mitigating Risks and Harm: By identifying potential safety concerns early, pharmacovigilance experts can work collaboratively with regulatory authorities and healthcare providers to implement risk minimization strategies. This may include updated labeling, prescribing guidelines, or safety warnings, reducing the likelihood of harm to patients.
- Preventing Adverse Drug Reactions: The early detection of potential safety signals helps prevent adverse drug reactions (ADRs) from occurring or worsening. This not only improves patient safety but also reduces the burden on healthcare systems caused by avoidable ADR-related hospitalizations and treatments.
- Enhancing Medication Safety Profiles: Signal detection contributes to a more comprehensive understanding of medication safety profiles. By continuously monitoring and analyzing safety data, pharmacovigilance experts can identify and evaluate potential risks associated with medications, leading to more informed decisions in drug development, prescribing, and patient management.
- Guiding Regulatory Actions: The detection of potential safety signals provides regulatory agencies with vital information to assess the benefit-risk profiles of medications. This informs decisions regarding drug approvals, label updates, and even market withdrawals, ensuring that only safe and effective medications are available to patients.
- Real-World Safety Evidence: Signal detection draws on real-world evidence, capturing safety data from diverse patient populations beyond the controlled environment of clinical trials. This real-world data provides a more accurate representation of the drug’s safety profile in routine clinical practice.
Future Trends in Signal Detection: Paving the Way for Advanced Pharmacovigilance
The future of signal detection in pharmacovigilance is full of promise as emerging technologies and innovative approaches shape the field. Advancements in big data analytics, artificial intelligence, and social media monitoring will revolutionize how potential safety signals are detected and analyzed. The integration of real-world evidence and wearable device data will provide a more comprehensive understanding of drug safety profiles, while pharmacogenomics will enable personalized medicine approaches. Embracing these future trends will empower pharmacovigilance experts to proactively ensure patient safety and well-being on a global scale. The key future trends includes:
Big Data and Real-World Evidence: Leveraging vast amounts of real-world data from electronic health records and patient registries for comprehensive signal detection and safety assessments.
Artificial Intelligence (AI) and Machine Learning (ML): Utilizing advanced algorithms to analyze large datasets, identify patterns, and expedite signal detection processes.
Social Media Monitoring: Tapping into social media platforms for insights into patient experiences and potential safety concerns that might not be captured through traditional reporting systems.
Wearable Devices and Remote Monitoring: Harnessing data from wearable devices and remote patient monitoring for real-time signal detection and adverse event monitoring.
Data Integration and Interoperability: Standardizing data collection and promoting interoperability to enable seamless data sharing and global collaboration in pharmacovigilance.
Pharmacogenomics and Personalized Medicine: Integrating pharmacogenomics to understand genetic influences on drug response and identify patient subgroups at higher risk of adverse reactions.
Conclusion: Embracing the Future of Signal Detection in Pharmacovigilance
As we embark on the journey towards the future of pharmacovigilance, the role of signal detection becomes increasingly vital in safeguarding public health and ensuring patient safety. With advancements in big data analytics, artificial intelligence, social media monitoring, and wearable devices, the landscape of signal detection is evolving at a rapid pace. These cutting-edge technologies offer a wealth of opportunities to revolutionize drug safety management and improve patient outcomes.
The proactive nature of signal detection enables early identification and assessment of potential safety concerns, allowing healthcare professionals and regulatory authorities to take timely action to mitigate risks. By embracing future trends and harnessing the power of real-world evidence, pharmacovigilance experts can gain deeper insights into the safety profiles of medications and make informed decisions that benefit patients globally.
As we look ahead, it is crucial to acknowledge the collaborative efforts of national and international pharmacovigilance centers and networks. Together, they foster data sharing, knowledge exchange, and collective expertise to strengthen signal detection capabilities worldwide.
At this juncture, we extend our heartfelt gratitude to each one of you, our valued readers, for joining us on this informative journey. Your commitment to drug safety and patient well-being is commendable, and your active participation in reporting adverse events is crucial to the success of pharmacovigilance efforts.
We invite you to stay connected with us as we continue to explore the ever-evolving field of pharmacovigilance and its impact on the pharmaceutical industry. By subscribing to our blog, you will receive regular updates on future articles, insightful case studies, and engaging discussions related to drug safety and healthcare advancements.
Together, let us pave the way towards a safer and healthier future, where pharmacovigilance plays a pivotal role in ensuring the well-being of all individuals who rely on life-changing medications.
Thank you for being a part of our journey, and we look forward to welcoming you back for more insightful discussions on pharmacovigilance and its contributions to public health.
Stay vigilant, stay informed, and stay safe!
Take an active role in drug safety. Report any adverse events or safety concerns to the FDA’s MedWatch program to help safeguard public health and contribute to a safer healthcare environment.
Subscribe to Pharmonly for the latest updates on drug safety and pharmaceutical advancements.
Guidelines for Pharmacovigilance
Continue reading series

Pingback: Guidelines for Pharmacovigilance and Global Collaboration
Pingback: Future of Pharmacovigilance - Advancements in Drug Safety