Ever thought why different kinds of packaging are available in medicines? What are these packaging materials made up of? What are the different types of packaging used in medicines? Why the packaging of a medicine is designed in that particular way? How to ensure the proper packaging of the medicines?
Well.. All these questions will be eventually answered in this post. All these questions belong to the topic the science of pharmaceutical packaging materials and the quality control of packaging materials.
The topic Quality control of Pharmaceutical Packaging materials has been divided into three parts. User can jump to selected part by clicking the part below.
- Pharmaceutical packaging materials
- Pharmaceutical packaging design
- Quality control tests for pharmaceutical packaging

Table of Contents
What is Quality control of Pharmaceutical Packaging materials ? Why is it needed?
As all the medicines are manufactured in pharmaceutical industry with lot of precautions, sterility and storage conditions on the top, these medicines needs to be packed in a specific packaging that maintains the integrity of the product. However, the physical and chemical attributes of the medicines decides the type of packaging to b needed. As per Food and Drug Administration (FDA) mandates, the package should be able to maintain the standards of identity, strength, quality, and purity of the drug for the whole shelf life period. WHO provides the guidelines for the quality control of pharmaceutical packaging materials.
Ideal properties of Pharmaceutical packaging
Although, the packaging and storage condition of the medication depends up on the type of medicine or molecule. Any packaging material should provide following features.
- Compatibility : It should not react with the pharmaceutical product
- Protection from environmental conditions: Including moisture, light, microbes, gases, and solvent losses
- Performance: It should be eco-friendly, economical, transit worthy, adaptable with common high speed packaging machines, and must meet tamper-resistant and tamper-evident requirements.
- Safety: Should be non-toxic and should not impart taste or colour to the product.
Packaging systems
A container closure system for a medicine contains primary packaging component and secondary packaging component. Primary packaging component remains in direct contact with the dosage form (e.g. tablets, syrups, and capsules). Typically, these elements shield the medication from the environment (e.g. moisture, gases, and light). Secondary packaging, on the other hand is not in direct contact with the dosage form and functions to protect and provide labelling for the primary container.
Pharmaceutical packaging materials
It is extremely important that the choice of container materials for any given product be made only after a careful analysis of the impact of these materials on the stability of the product and of the effectiveness of the container in protecting the product during extended storage under varying environmental conditions of temperature, humidity, and light. Commonly employed packaging materials include glass, metal, rubber, and plastic.
Glass
As it possess good protective properties and is available in wide range of size and shapes, glass is a commonly used as packaging material. Its property of not being deteriorated with time and protection from every external element except light makes it a good choice for packaging. However, fragility, expensive machinability, and weight contributes to its disadvantages too.
The Composition of glass include Sand, Soda ash, Limestone, and cullet. Cullet (broken glass) is added to the mixture to fuse all the contents. Addition of different element provides different nature to the glass. Reduction of the sodium in glass makes it chemically resistant but at the same time making it hard and expensive to melt. Boron oxide is used to aid melting process that acts by reducing the temperature required. Similarly, lead provides brilliance and clarity to the glass, while alumina provides chemical resistance, hardness and durability to the glass.
Manufacturing of glass containers is done by four processes that include blowing, drawing, casting, and pressing. Compressed air is used to blow molten glass into a metal mold’s hollow. Molten glass is drawn through dies or rollers in order to form it. Commercially produced rods, tubes, sheet glass, and other objects of consistent diameter are typically made via drawing. In pressing, molten glass is pressed against a mold’s side using mechanical force. In casting, molten glass forms in the mold chamber due to gravity or centrifugal force.
Types of glass
Type I, Type II, Type III, and Type NP of glass are described in the USP and NF, which also offers tests to determine a glass’s chemical resistance. These tests determine how much alkalinity pure water extracts from glass under well regulated, high temperature circumstances.
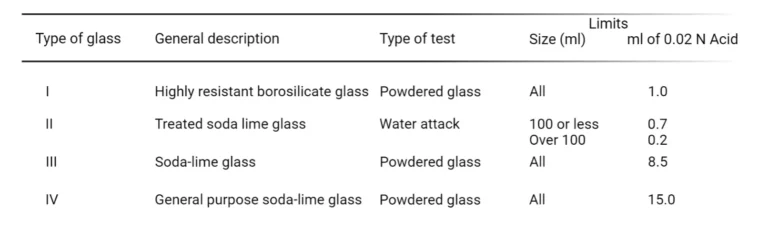
Irrespective of the type of glass, USP XX has acknowledged the possibility of release of alkali ions from each of four glass types. In order to prevent that, many approaches have been attempted by modifying the manufacturing processes.
Manufacturing process modifications during glass manufacture
- The sodium oxide used in the glass manufacturing in replaced with other alkali oxides to reduce the alkali content release.
- Treating the glass surface to water vapor and sulfur dioxide at elevated temperature that results in the formation of thin positively charged hydrogen ion that renders glass surface resistant to the attack of water.
- Iron and manganese oxides are added to the glass to impart amber color that prevents the photosensitive drugs.
- The oxides present in glass can lead to flake formation b reacting with drugs. This is prevented or delayed by pretreating containers with dilute acid.
Plastic
The simplicity with which they may be manufactured, the high quality, and the design flexibility of plastics have all contributed to their success in the packaging industry. Because plastic containers are so resistant to breaking, they provide consumers with safety as well as a decrease in breakage losses at all stages of distribution and use.
Various additives and one or more polymers make up plastic containers. Antioxidants, antistatic agents, pigments, impact modifiers, lubricants, plasticizers, and stabilizers are a few examples of additives that can be used with plastic containers in general. The structures of various monomers used to build up plastic containers, along with their properties is depicted in image below.

Drug - plastic consideration
The problem of permeation has also been observed and depends upon the type of plastic material (polymer) being used for the manufacturing of container. The permeation can occur from preparation to the plastic container, leaching out of plastic contents from the container o the formulation, permeation of content from atmosphere to preparation through container, and absorption or adsorption of preparation onto the container surface. Moreover, the plastic container can react with the formulation chemically or physically leading to container deformation. Things to be considered while selecting appropriate plastic container are discussed below.
- Permeation: Permeation involves the transmission of gases, vapors, and liquids from atmosphere to the preparation through the container. This may affect the formulation by inducing microbial growth, hydrolysis, oxidation and other reactions in susceptible formulations. The permeation property of a container depends upon the material used for manufacturing. Hydrophilic material such as nylon imparts poor barrier to water vapors while hydrophobic one provides better protection. However, water-in-oil emulsions are unstable in hydrophobic-made containers due to leaching of oil phase into the container.
- Leaching: It can be defined as the leaching-out of the contents such as coloring agents, or other additives from the plastic container to the formulation which can make the formulation toxic.
- Sorption: It is the removal of drug product constituents by the plastic packaging material. The constituent can be active pharmaceutical ingredient or excipient including preservative. Chemical structure, concentration of active ingredients, pH, temperature, length and area of contact, and solvent syste are some factors that influence sorption.
- Chemical reactivity: The property of any formulation component should not has chemical reactivity towards the plastic container. Sometimes, even micro quantities of chemicals may react with the container.
- Modification: As the term indicates, it includes deformation of the plastic container structure due to any of the above discussed phenomenon such as sorption, leaching, etc. Modification in the structure of polyethylene plastic containers has been noted with gases and vapors, oils, and surface active agents. Similarly, Polyvinylchloride (PVC) based containers gets hard due to extraction of plasticizer from them by the solvents.
Metal
Metal containers are incredibly durable and unbreakable. Metal enables relatively simple fabrication and usage for applications needing malleability, such as collapsible tubes. Dispersible systems can be easily stored in collapsible metal tubes and have the viscosity of a soft paste, gel, cream, or ointment. Tin, tin-coated lead, plastic-coated tin, aluminium, and steel are among the metals frequently utilized for such tubes.
Tin
Food, medicine, and other products whose cleanliness is of utmost importance should be packaged in tin. Aerosol cans are commonly made by electroplating tin onto sheet steel to increase corrosion resistance and make soldering easier. Of all the metals used in collapsible tubes, tin is the most chemically inert. It has a nice aesthetic and works with many different products. Due to their non-reactive qualities, tin and tin-coated tubes are typically used, though it has been shown that they can corrode under chloride or acidic environments.
Aluminium
Aluminum foil can be formed into rigid containers, semirigid containers, blister construction, or laminates. Aluminum tubes offer significant savings in product shipping costs because of their lightweight. They provide the attractiveness of tin at somewhat lower cost. Coated and uncoated aluminum tubes are being used, but are not always satisfactory. The application of an epoxy lining to the internal surfaces of aluminum tubes was found to make them more resistant to attack.
Lead
The most affordable metal, lead is frequently used in non-food items like adhesives, inks, paints, and lubricants. Due to the possibility of lead poisoning, lead should never be utilized alone in anything that will be consumed internally. Lead tubes with internal linings are used for things like fluoride toothpaste.
Linings
Although resins or lacquers are often sprayed on, wax-type formulations or resin solutions can be used to flush the interior if the product is incompatible with bare metal. Compared to the uncoated, an epoxy liner costs around 25% more. With water-base goods in tin tubes, wax linings are most frequently employed. Phenolics, epoxides, and vinyls, which offer better protection than wax but are more expensive, are used with aluminium tubes. Epoxides offer superior protection against alkaline goods whereas phenolics work well with acidic products.
Rubber
Natural rubber is vulnerable to oxidizing agents, oils, benzene, and ketones but is resistant to weak mineral acids, alkalies, and salts. Handling acids, especially diluted aqueous solutions, typically involves the use of hard rubber, which is created by adding 25% or more sulphur to natural or synthetic rubber. Because synthetic rubbers outperform natural rubber in many ways, including resistance to oxidation, solvents, oils, and various chemicals, synthetic rubbers like polychloroprene (also known as Neoprene), polysiloxanes (also known as Silicon rubber), polyisoprene, polybutadiene, chlorinated polyethylene elastomer, epichlorhydrin elastomer, styrene-butadiene rubber, synthetic rubbers are becoming of great importance. In pharmaceutical packaging, rubbers are used as stoppers, cap liners, and parts of dropper assemblies.
Rubber stoppers in touch with the liquid in the vial run the risk of absorbing active ingredients, antibacterial preservatives, or other substances, as well as having one or more rubber components extract into the vial solution. The application of epoxy lining on the stoppers has noted to decrease the leaching out of extractives from rubber to water for injection. Moreover, the sorption of the preservatives from the preparation on to the rubber can be prevented by teflon coating of rubber.
The topic quality control of pharmaceutical packaging materials has been continued further. Please read.

Get all Pharmacy books for FREE
Free downloadable PDF's of all PCI recommended books are provided.
