Welcome to the realm of pharmacovigilance, where data management and reporting emerge as guardians of medication safety. In this journey, we unravel the dynamic interplay of data, vigilance, and patient well-being.
Through the synergy of vigilant healthcare professionals, engaged patients, and advanced analytics, data management transcends the mundane. It transforms into a sentinel that uncovers patterns, unearths concerns, and shapes safer medical landscapes.
Join us as we explore the corridors where data evolves into insights, decisions, and better outcomes. Your engagement ignites a brighter future for drug safety.
Before that, If you’ve just joined us, be sure to catch up on our previous post by clicking here where we delved into the intricacies of pharmacovigilance regulations and the power of global collaboration. In this installment, we continue our exploration by peering into the world of pharmacovigilance data management and reporting. Let’s dive in and uncover how data transforms into insights that shape the safety of medications for all.
Table of Contents
The Backbone of Pharmacovigilance Data
In the intricate web of pharmacovigilance, data serves as the foundational thread that weaves together the fabric of medication safety. Adverse event reports, patient demographics, and clinical particulars comprise this intricate mosaic, fostering a comprehensive understanding of potential risks and benefits.
Vigilant healthcare professionals, through their astute observations, contribute invaluable pieces to this puzzle. Patients, too, play a pivotal role by sharing their experiences, providing essential insights that guide future medical practices. Together, these elements form the backbone of pharmacovigilance, shaping a proactive approach to ensuring drug safety.
Every data point, meticulously gathered and cataloged, holds the potential to unravel critical safety signals. As we traverse this data-rich landscape, we’ll uncover how its strategic management and insightful reporting lead us towards a future of safer medications and enhanced patient care.
Streamlined Pharmacovigilance Data Management: Organizing for Precision
In the labyrinth of pharmacovigilance, efficient pharmacovigilance data management is not just a convenience; it’s a necessity that ensures the integrity and accuracy of safety insights. The sheer volume and complexity of safety data demand structured systems that can adeptly handle, organize, and retrieve information.
Modern pharmacovigilance data management leans on advanced databases and specialized software solutions to tame the deluge of data. These tools provide a secure haven for storing vast repositories of adverse event reports, patient records, and related information. Their role extends beyond storage – they empower quick retrieval and systematic analysis, enabling pharmacovigilance professionals to identify patterns and potential risks.
By implementing organized pharmacovigilance data management systems, pharmacovigilance practitioners pave the way for precision and efficiency. As we journey through this section, we’ll uncover how these systems harmonize data, expedite decision-making, and ultimately enhance patient safety.
Insights from Reporting: Unearthing Patterns
The symphony of pharmacovigilance reaches its crescendo through the collective reporting efforts of vigilant healthcare professionals and engaged patients. Adverse event reporting is not just an administrative task; it’s a conduit through which potential safety concerns surface and trends emerge.
Healthcare professionals, armed with their clinical acumen, provide a wealth of first-hand observations. Patient reports, capturing experiences beyond clinical settings, offer unique perspectives. These inputs intertwine to create a comprehensive narrative of medication safety.
The process of reporting unravels hidden patterns that might otherwise stay concealed. Emerging trends, subtle correlations, and potential signals come to light, guiding further investigations. Through this collaborative reporting ecosystem, pharmacovigilance professionals gain insights that inform regulatory decisions, ultimately safeguarding public health.
As we explore this realm, we’ll illuminate the intricate dance between reporting and analysis, understanding how each report contributes to a larger symphony of drug safety.
The Power of Analytics: Detecting Signals and Assessing Risks
The realm of pharmacovigilance transcends raw data; it’s about transforming data into actionable insights through the alchemy of analytics. Data analysis is the torchbearer that illuminates the path toward detecting potential safety signals and assessing associated risks.
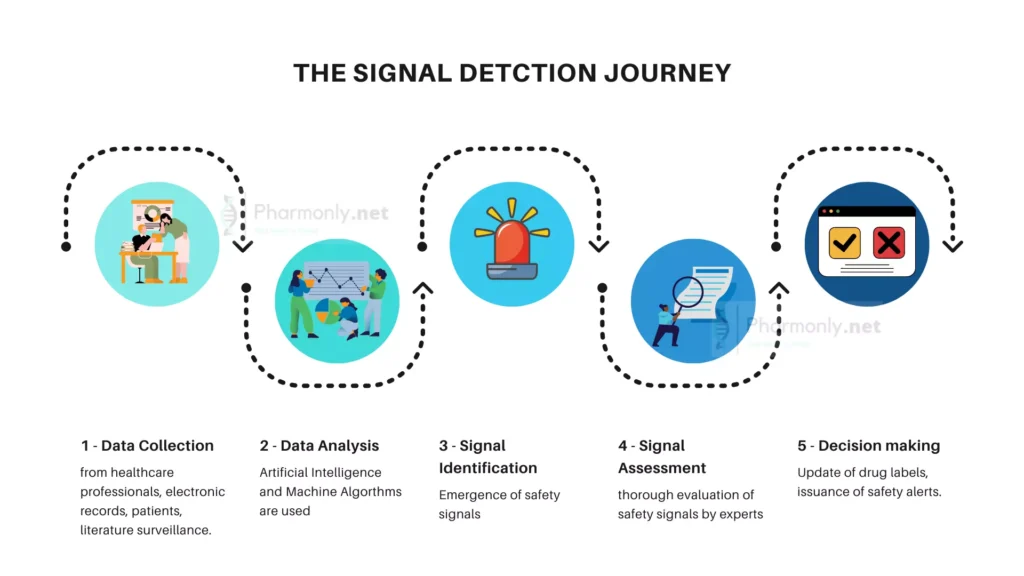
Data Analysis for Signal Detection: Unlocking Insights
In the sea of data, seemingly disparate elements converge to reveal meaningful patterns. Statistical methods and algorithms play a pivotal role, sifting through vast datasets to discern anomalies and deviations. By identifying these deviations, pharmacovigilance experts unmask potential signals that warrant further investigation. These signals serve as early warnings, offering a chance to evaluate and respond swiftly to emerging safety concerns.
Risk Assessment: Navigating Uncertainties
Statistical analysis doesn’t stop at signal detection; it also extends to risk assessment. By analyzing historical data, experts gauge the likelihood of adverse events and their potential impact. This assessment aids in prioritizing resources, targeting surveillance efforts, and guiding regulatory decisions.
Navigating Complexion with Precision
In the intricate dance of pharmacovigilance, data analysis wields the power to decipher complexity. Statistical methods and algorithms stand as the guiding stars, navigating through the vastness of data to unearth signals and assess risks. Together, they empower pharmacovigilance professionals to uphold medication safety with utmost precision.
Real-time Monitoring of Alerts: Swift Responses for Safer Medications
In the modern era of pharmacovigilance, the static model of retrospective analysis is giving way to a dynamic paradigm – one powered by real-time monitoring and instant alerts. This evolution signifies a quantum leap in our ability to respond swiftly to emerging safety signals, ensuring medications remain a source of healing rather than harm. The typical sample dashboard of real-time monitoring of pharmacovigilance data management is shown in below figure.
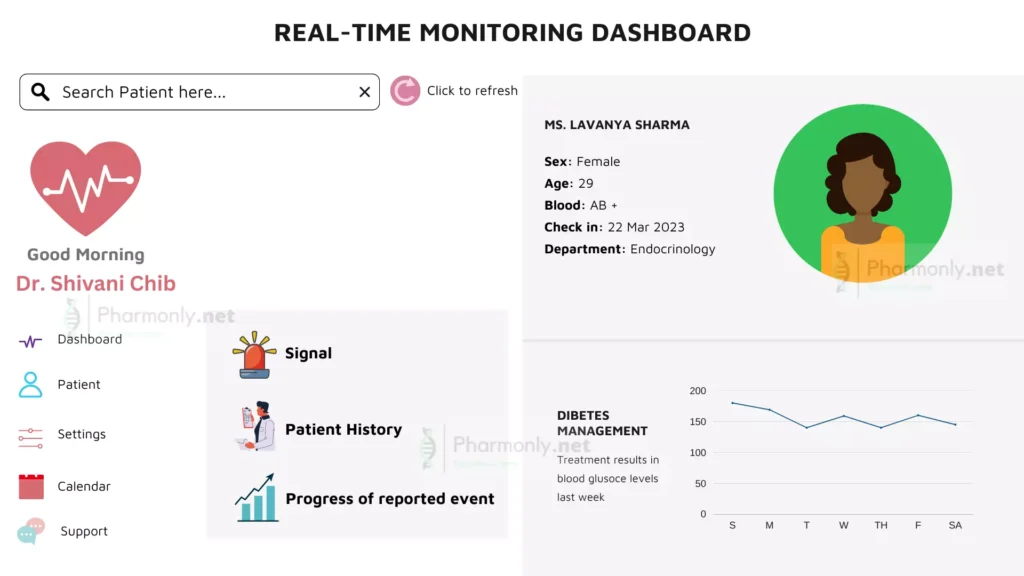
Unleashing the Power of Real-time Data
Real-time monitoring harnesses the potential of connected systems and digital infrastructure. As data flows in from diverse sources, it’s processed and analyzed almost instantaneously. This continuous stream of information allows us to cast a proactive net, detecting even subtle changes in safety profiles.
From Alerts to Actions: The Timely Intervention
The heart of real-time monitoring lies in its ability to trigger alerts at the slightest hint of anomalies. These alerts act as sentinels, prompting pharmacovigilance experts, regulatory bodies, and healthcare professionals to initiate immediate investigations. This swiftness in response is instrumental in preventing potential harm before it escalates.
Elevating Patient Safety with Speed
The significance of real-time monitoring extends beyond data streams and alerts. It’s about elevating patient safety through proactive measures. By identifying safety concerns at their nascent stages, we empower healthcare stakeholders to take informed actions that safeguard public health.
A New Era in Drug Safety
Real-time monitoring ushers in a new era in drug safety – one characterized by agility, foresight, and a dedication to minimizing risks. As we navigate this transformative landscape, we glimpse a future where timely interventions mitigate harm, and medications continue to be agents of healing.
Conclusion: Shaping a Safer Tomorrow through Data-driven Vigilance
As we draw the curtains on our exploration of pharmacovigilance data management and reporting, a resounding truth emerges – the power of data is the compass guiding us toward safer medications and healthier lives. This journey through data’s corridors has unveiled the intricate dance between vigilant healthcare professionals, engaged patients, and cutting-edge analytics.
In a world where information evolves into insight, each adverse event report and data point becomes a piece of a puzzle that, when connected, forms a picture of medication safety. From organized pharmacovigilance data management systems to real-time monitoring, the steps we take today shape the path toward a future where potential risks are intercepted early, interventions are swift, and patient well-being is paramount.
Let’s continue to embrace the principles of data-driven pharmacovigilance, championing a culture of reporting, analysis, and collaboration. Through our collective efforts, we carve out a landscape where medications heal, protect, and uplift – truly embodying the essence of healthcare’s noble mission.
Thank you for joining us on this enlightening journey. As we bid adieu to this chapter, we invite you to stay connected, for our future posts will unravel more layers of drug safety and illuminate the path ahead.
Your voice matters in the realm of pharmacovigilance. Share your thoughts and experiences in the comments below. By joining the conversation, you contribute to the collective knowledge that shapes safer medication practices. Elevate your role in ensuring medication safety by becoming part of the MedWatch program. Stay informed and engaged – subscribe to our newsletter for updates on upcoming posts that continue to unravel the intricacies of drug safety.
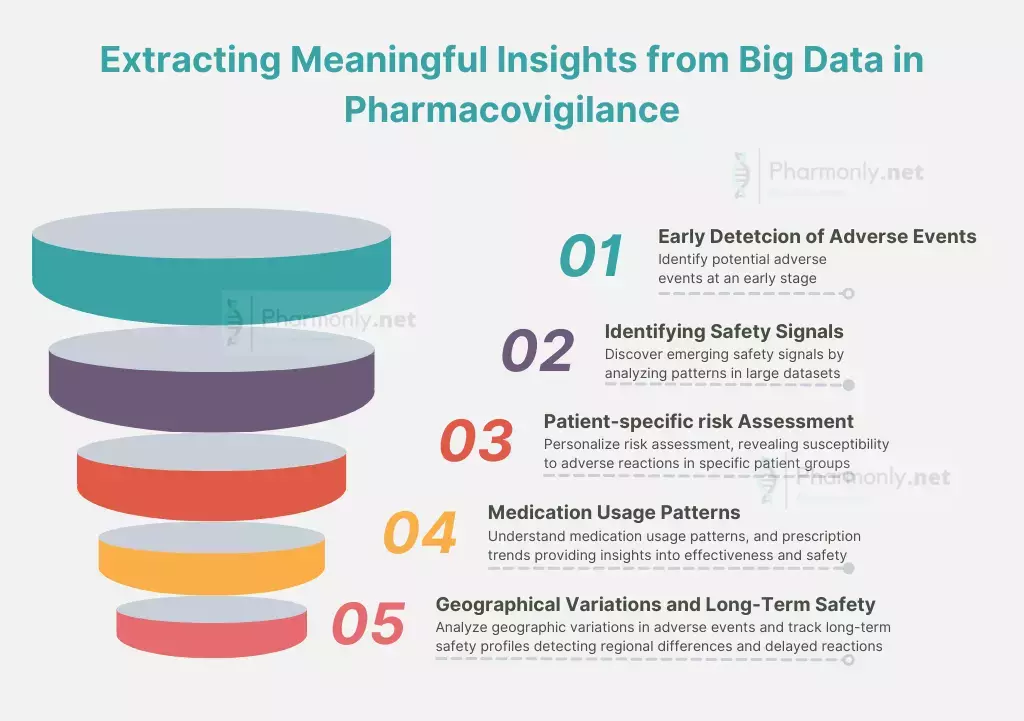
Explore Future of Parmacovigilance
Continue reading series...

Pingback: Future of Pharmacovigilance - Advancements in Drug Safety
I?¦ve learn a few excellent stuff here. Definitely value bookmarking for revisiting. I surprise how so much attempt you set to create any such great informative web site.